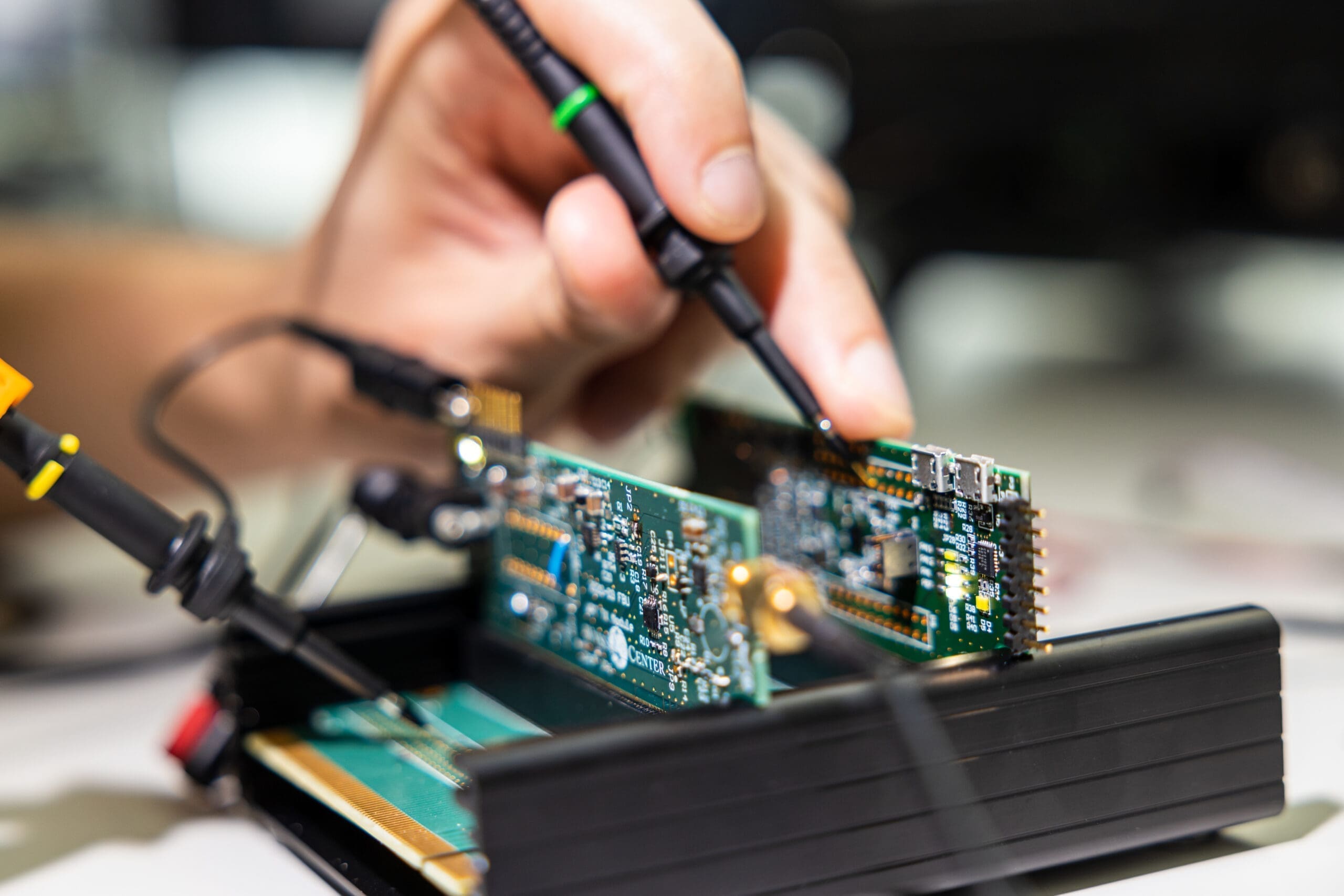
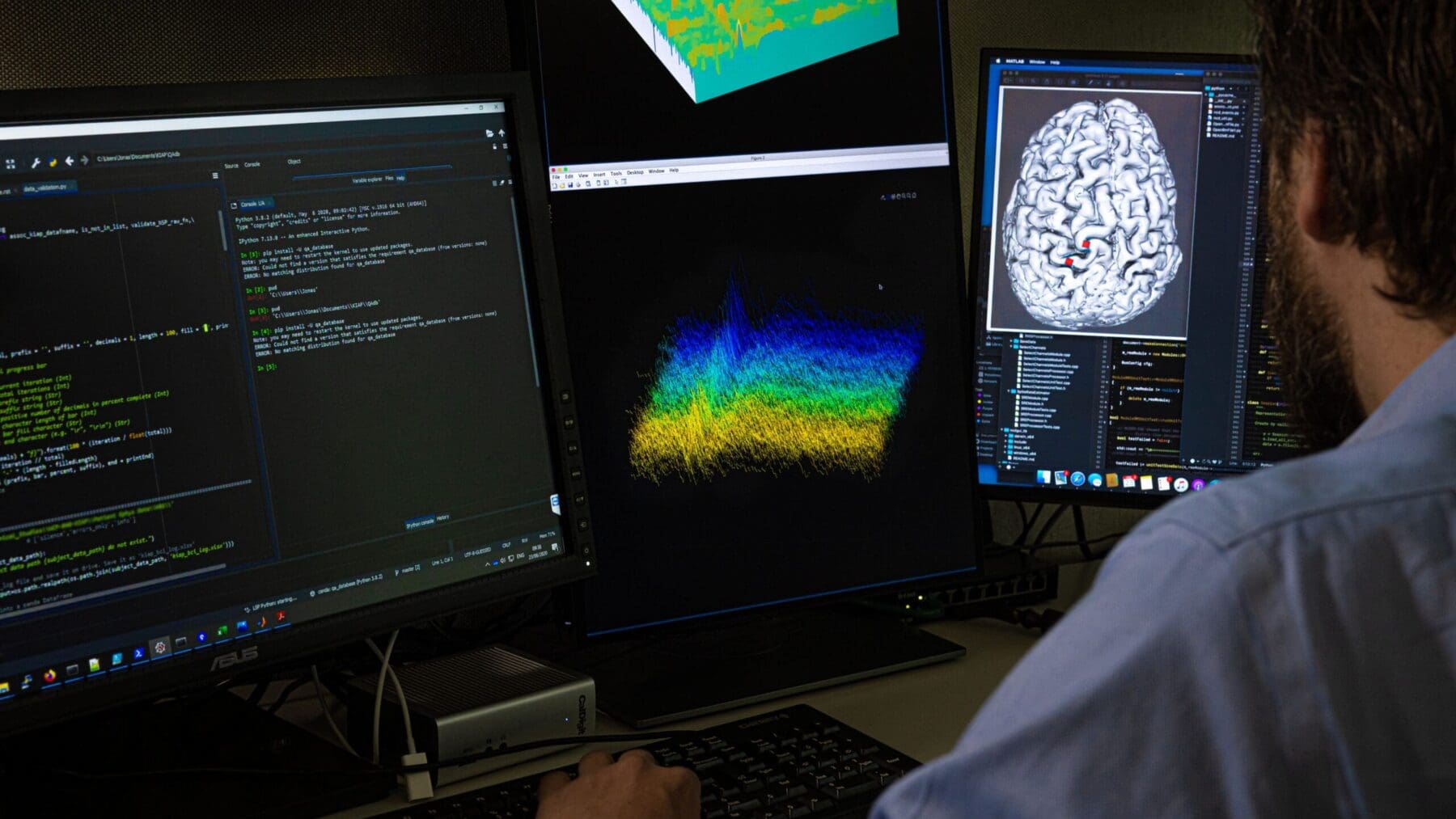
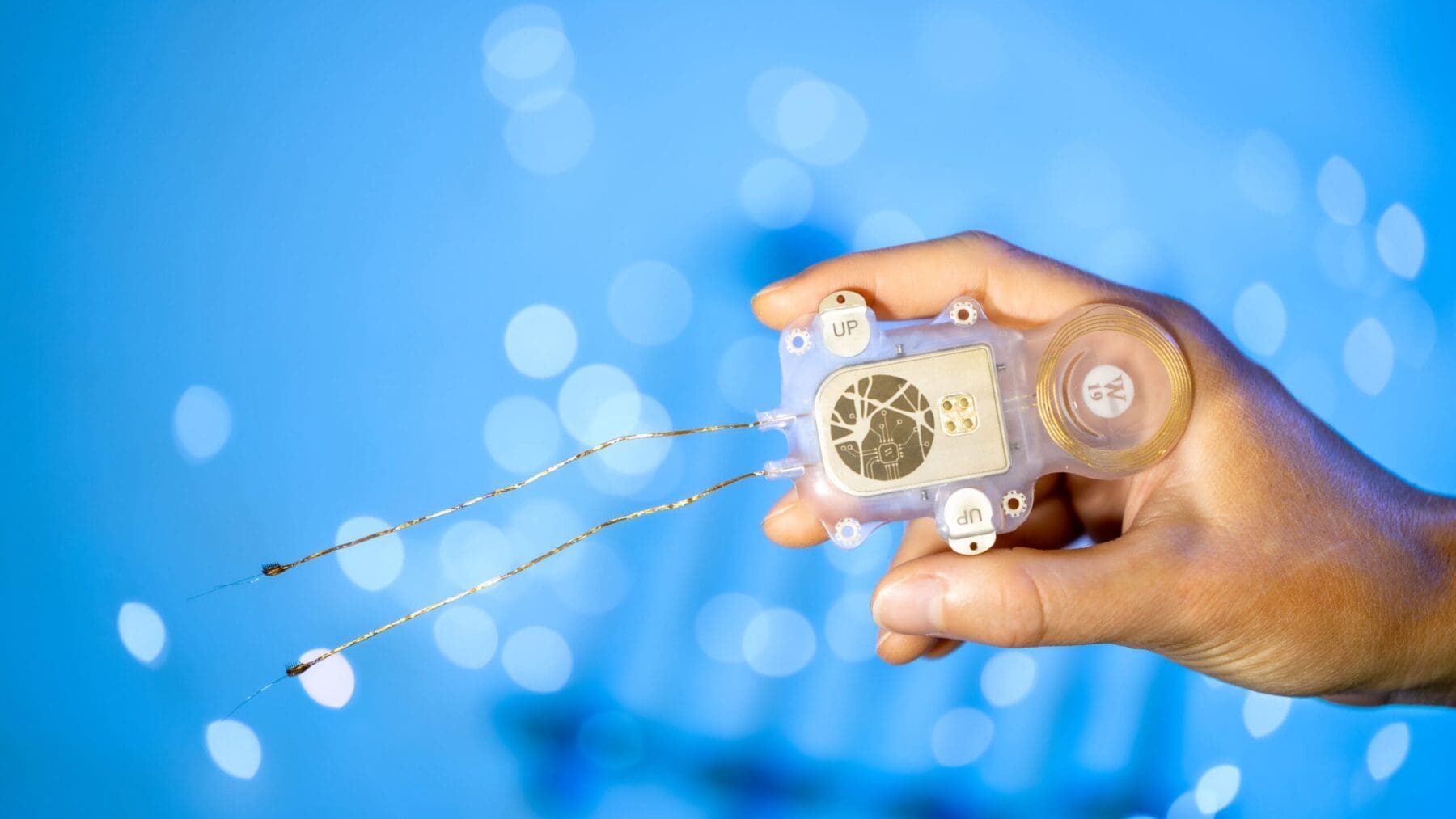
Our capabilities drive the development of technologies and devices that can access and directly interface with the human brain. The aim is to either record brain signals for improved communication or movement control through intelligent prostheses and/or modulate the brain’s activity via electrical, optical or biological means for therapy.
Our development efforts depend on close collaborations with clinical and academic leaders in the field of neuroscience and neurosurgery and take place within the strict ethical and regulatory frameworks in Switzerland, the EU and the US.

Key focus areas in neurotechnology development
The Wyss Center’s development efforts begin at the idea generation stage and continue with prototype fabrication and on to product development and clinical validation.
We develop innovative medical grade technologies that interface with the nervous system such as brain-machine interfaces, neuromonitoring and neurostimulation devices to understand, repair or replace nervous system function. Leading indications include epilepsy, Parkinson’s disease, ALS, spinal cord injury and stroke.
Our work spans the following aspects of development:
Concept – Conceptualization of innovative technologies that interface with the nervous system
Feasibility studies – Readiness screening and technical, clinical and business feasibility assessments including business model development and reimbursement scenarios
Prototype development – Design and prototyping of hardware and software subsystems and their integration
Validation – Testing, analysis, modeling and optimization in the laboratory and the clinic
Quality assurance – Providing confidence that all quality requirements are fulfilled according to European and US standards, technical documentation generation and review
Regulatory strategy and submission – Development of regulatory strategies as a technology is developed, technical documentation generation and review according to applicable EU and US standards and regulations
Clinical trials – Clinical trials protocol design and submission, collaboration with clinical groups for first-in-human studies
Commercialization – Licensing of technologies developed at the Wyss Center to commercial entities for the benefit of humankind