Alzheimer’s disease: an alternative hypothesis based on synaptic alterations
Human hippocampal brain section from an Alzheimer’s disease patient showing amyloid precursor protein (green) clustering around amyloid plaques (purple and red). © Gaël Barthet
New research suggests that targeting proteins essential to neurotransmission could be a promising alternative to treat Alzheimer’s disease
New research published today in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association could explain why neurons fail to communicate effectively in people with Alzheimer’s disease (AD). The study conducted by researchers in the laboratory of Christophe Mulle at the Interdisciplinary Institute of Neuroscience in Bordeaux opens a new research path to establish the molecular mechanism causing AD.
Despite intense efforts in clinical research, there is no treatment to cure or even slow down the progression of AD. Current clinical interventions are typically based on the ‘amyloid cascade’ hypothesis, postulating that neurodegeneration in AD is caused by the abnormal accumulation of amyloid plaques in the brain. However, these interventions have failed to demonstrate clinical efficacy. Recently, the European Medicines Agency did not approve a controversial new drug for AD that targets plaques, concluding that the benefits do not outweigh the risks. The decision leaves an estimated 8 million people living with dementia in the EU with no treatment options, highlighting the urgent need for an alternative target for AD research.
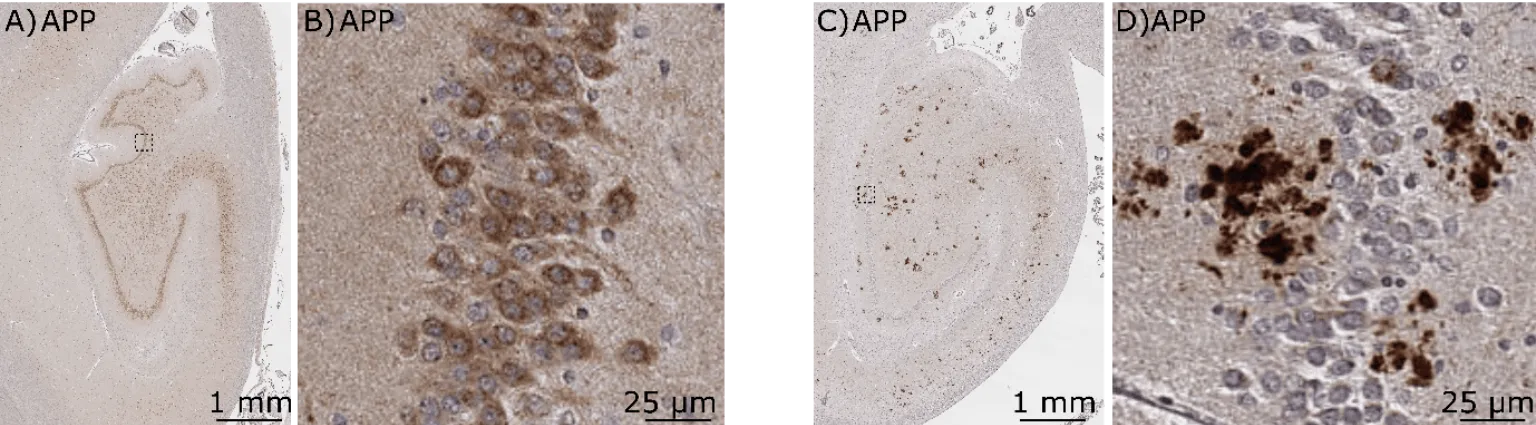
The new study reveals that the amyloid peptide composing the plaques is not the only offender accumulating in the human AD brain. Its precursor, the amyloid precursor protein (APP), was found to surround amyloid plaques in intense microscopic staining.
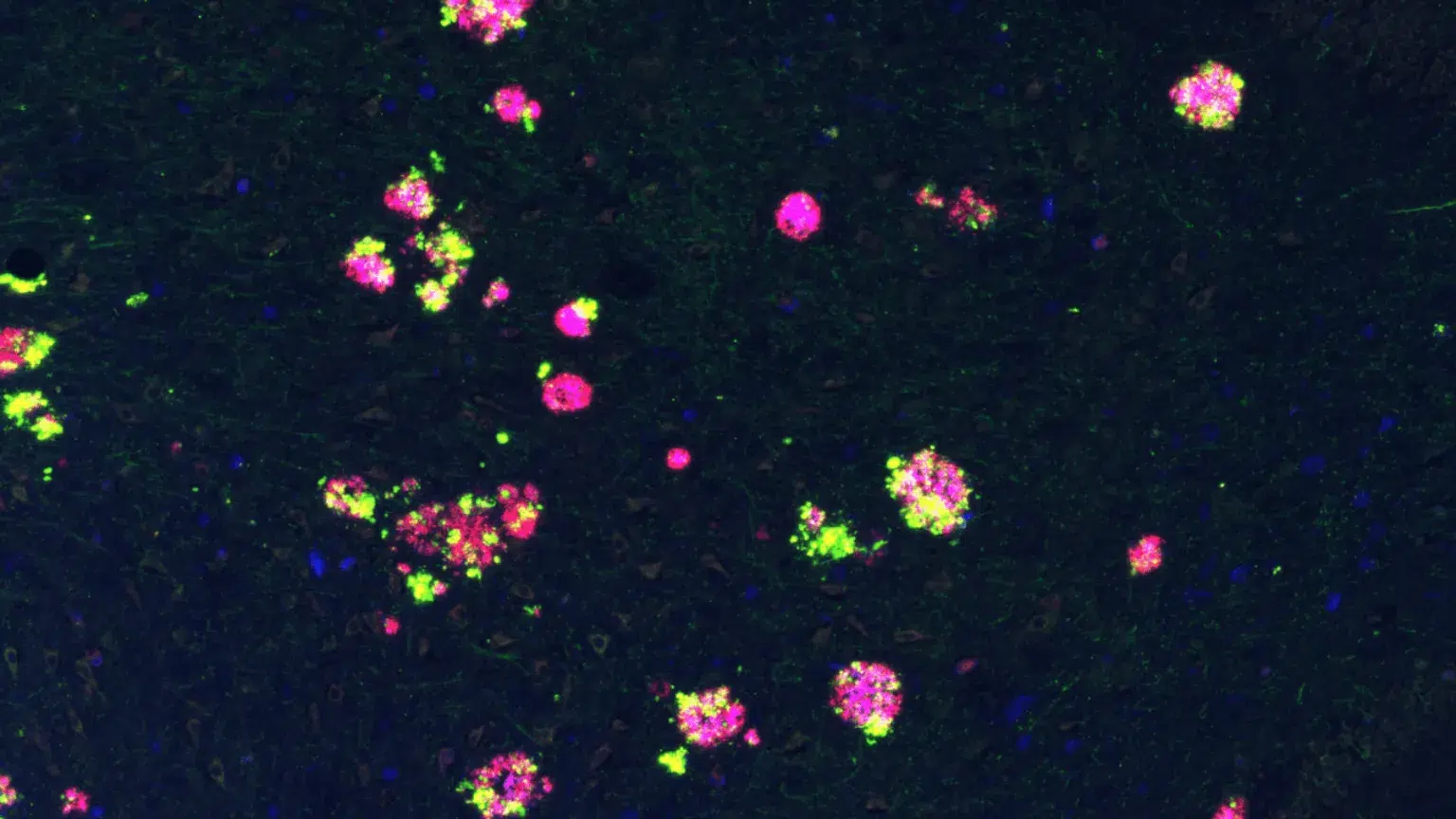
“Remarkably, our research revealed that the areas of the brain where APP accumulates, contain abnormal amounts of proteins essential for the communication between neurons,” said Dr Gaël Barthet senior author on the paper, now Director of Neuroscience at the Wyss Center.
The communication between neurons occurs at junctions called synapses where neurotransmitters released by a pre-synaptic neuron cross the junction to activate a post-synaptic neuron.
“What we found fascinating was that APP accumulated with an excess of presynaptic proteins whereas post-synaptic proteins were depleted, indicating severe impairment of neuronal communication at these sites,” said Dr Tomas Jorda, lead author of the paper and Post-Doctoral Research Associate in neurobiology at the University of Geneva.
“The new findings introduce a new direction for us to explore using our innovative, multi-scale, multi-modal approach at the Center,” said Dr Richie Kohman, Chief Scientific Officer at the Wyss Center.
The Wyss Center’s translational research on neurodegenerative diseases uses novel molecular labelling techniques coupled with advanced microscopy and data analysis tools to examine specific neuronal populations, synaptic markers, and single-cell transcriptomics in the human brain. These innovative imaging approaches enable the study of the human brain in large sections or even in entire 3D samples. The Wyss Center is using this pipeline to investigate the molecular mechanisms at play in neurological and psychiatric disorders, to establish early biomarkers and identify new therapeutic targets.
The paper, ‘APP accumulates with presynaptic proteins around amyloid plaques: a role for presynaptic mechanisms in Alzheimer’s disease?’ is published in Alzheimer’s & Dementia 2022 DOI: 10.1002/alz.12546
Director of Neuroscience
gael.barthet@wysscenter.ch
+41 58 201 03 67
Wyss Center for Bio and Neuroengineering
Chemin des Mines 9, 1202 Geneva, Switzerland
Christophe Mulle
Research Director at CNRS
christophe.mulle@u-bordeaux.fr
Team ‘Synaptic Circuits of Memory’
Interdisciplinary Institute for Neuroscience, CNRS Université de Bordeaux
Bordeaux, France