Repairing the spinal cord
Gene therapy to boost rehabilitation success following spinal cord injury

Goal | Gene therapy for spinal cord injury
Status | Completed
Timeframe | 2022 – 2023
Area of Research | Neurovisualization technology
Partners | CHUV, EPFL, Neuro Restore, FCBG
Lead | Grégoire Courtine
Gene therapy to boost rehabilitation
The collaborative project is designing a gene therapy that can be combined with neuroprosthetic rehabilitation to improve recovery, restore walking and enable people to restart daily activities after spinal cord injury.
Globally there are estimated to be more than 20 million people living with a spinal cord injury (SCI) and nearly a million new cases each year. SCI interrupts the flow of information between the brain and the rest of the body and can result in permanent paralysis, loss of sensation and an inability to regulate functions like breathing and blood pressure, seriously diminishing quality of life. Despite important research progress, there is still no clinically available solution to repair the spinal cord.
Coaxing neural repair
Although some natural repair occurs following incomplete SCI, when neurons rewire around the injury, there is no natural regrowth after complete SCI. Establishing a neural bridge across the injury site, and training the new neural connections to integrate with existing circuits below the injury, remains a major hurdle after anatomically complete SCI. Promising gene therapy strategies that address these challenges are now at the pre-clinical research stage.
A gene therapy to regenerate the spinal cord
The collaborative Wyss Center, CHUV and EPFL team is drawing on expertise in gene therapy, advanced microscopy and neurosurgery as well as spinal cord stimulation and rehabilitation to design a therapy that can be delivered and combined with neuroprosthetic rehabilitation to improve recovery, restore walking and enable people to restart daily activities after spinal cord injury.
Reawakening genes for growth
Neurons grow during embryonic development when the spinal cord is formed. After birth, the genes driving this growth switch off. If reactivated, they can induce neuronal regeneration after injury, even in adults. In collaboration with the Bertarelli Foundation Gene Therapy Platform at Campus Biotech, the team is developing a gene therapy using growth stimulating factors to recapitulate the natural developmental process during recovery from SCI. The team aims to use this approach of developmentally inspired repair to selectively and safely target neurons in the spinal cord and guide them across the site of complete SCI.
The approach requires three crucial factors:
- The intrinsic growth capacity of neurons which is reactivated by the gene therapy.
- A structurally supportive substrate or scaffold to help bridge the gap left by injury.
- Chemo attractants to encourage neurons to grow across the site of the injury until they contact healthy tissue.
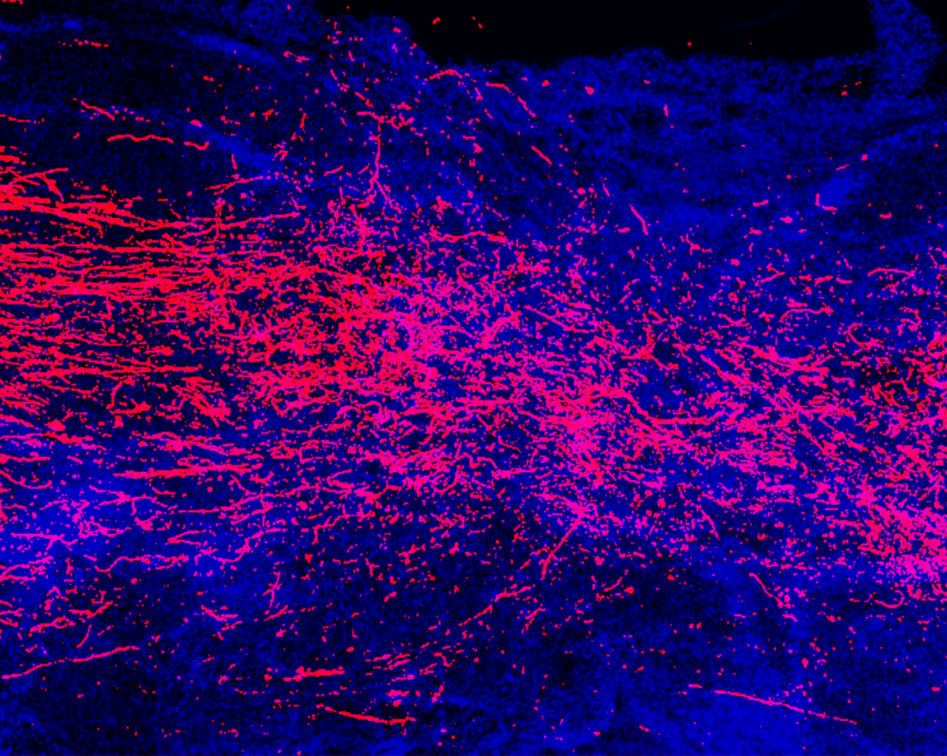
Observing neural repair with lightsheet microscopy
The team is using the advanced imaging pipeline at the Wyss Center to prepare, fluorescently label, image and analyze the entire central nervous system including the brain and spinal cord, in mice.
The state-of-the-art, custom-built, lightsheet microscopes reveal the cellular characteristics of specific neural circuit repair in three-dimensions at high resolution allowing the team to catalog the molecular choreography of recovery from spinal cord damage with exquisite detail.
While gene therapies are not yet in regular clinical use, the approach is quickly evolving and likely represents the future of regenerative medicine. If successful, this therapeutic strategy would present a promising treatment for spinal cord injury, and may have implications for other central nervous system disorders and diseases.