Finding cures for the future in the brains of the past
Brain mapping: Uniting multi-scale brain data to reveal disease mechanisms

Goal | improving data to map the brain
Status | Completed
Timeframe | –
Area of Research | Neuro visualization technology
Partners | HUG, UNIGE
Lead | Christophe Lamy
Evolution | spun out as DataFlight Solutions Ltd.
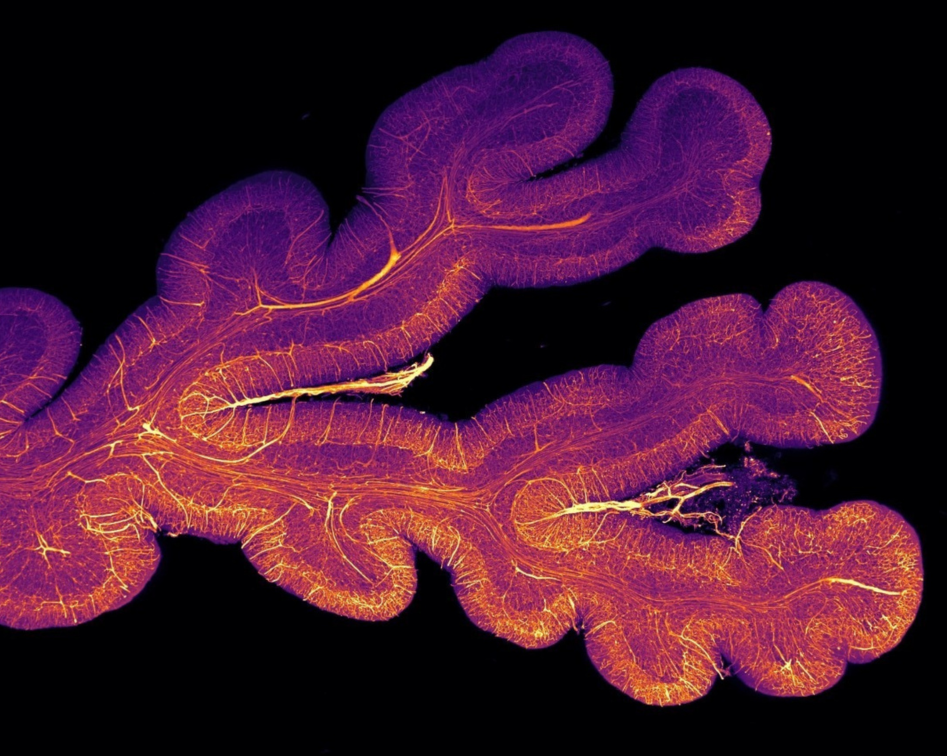
The challenge
In the past it has been difficult to reconcile advances made at a cellular level with a whole-brain approach. This collaborative project combines cellular resolution data with whole-brain information to provide genuinely new insights into the pathological mechanisms underlying human brain disorders.
For decades the microscopic study of brain tissue has helped pathologists understand disease, describe the cellular diversity of the nervous system and define specific features of brain disorders. In parallel, neuroimaging methods have revealed the function and dysfunctions of whole brain neural circuits. However, technical limitations of traditional microscopy have made it difficult to relate cellular level advances to whole brain observations. Our approach unifies neuroscience across scales, from genes to the whole brain, with the goal of revealing the mechanisms of brain disease, establishing diagnostic biomarkers and identifying new therapeutic targets.
Pioneering 3D maps of the brain
This collaborative project works closely with the Geneva Brain Collection and also uses healthy and diseased human brain tissue from other sources. Advances in imaging techniques, particularly lightsheet microscopy present at the Advanced Lightsheet Imaging Center (ALICe), enable the imaging of much larger samples while retaining their structure.
The team will develop a next-generation histopathology approach taking advantage of novel molecular labelling techniques coupled with advanced microscopy and data analysis tools, to explore the relationship between cellular and whole brain mechanisms in both healthy and diseased tissue. It will initially focus on neurodegenerative dementia and eventually explore the broad range of conditions contained in the GBC.